Low Grade Chronic Inflammatory Response in Pathogenicity of Diabetes Mellitus
CURRENT RESEARCH IN DIABETES & OBESITY JOURNAL JUNIPER PUBLISHERS
Authored by Shamim Shaikh Mohiuddin
Abstract
Acute phase reaction which is mainly cytokine-mediated is observed to be closely involved in the pathogenesis of type 2 diabetes. Since maximum world populations are at elevated risk of developing diabetes, we tested this hypothesis by estimating circulating acute phase proteins in type 1(T-1), type 2 (T-2) diabetic patients and type 2 diabetic patients under oral hypoglycemic drugs for duration of at least 5 years.
The acute phase proteins, α1- antitrypsin, α1- acid glycoprotein, ceruloplasmin and fibrinogen were estimated in the plasma in newly diagnosed 12 T-1 cases, newly diagnosed 25 T-2 cases and 25 T-2cases under oral hypoglycemic agent for at least 5 years Thirty normal controls to match the age and sex of the test groups were also studied. The levels of these proteins were correlated with their BMI and random plasma glucose values.
In comparison with the controls, the values of all the four proteins studied were significantly elevated in T-1 patients and T-2 patients (p<.00001) and reduced significantly except α1- acid glycoprotein and ceruloplasmin. Interestingly, no correlation was found with BMI or the degree of hyperglycemia in either of the types. A low grade inflammatory process is definitely implicated in the pathogenesis of type 2 diabetes and even type 1 diabetic patients. This line of pathological basis should be further explored for diagnosis, management and follow up.
Keywords: Chronic inflammation; Diabetes mellitus; Acute phase reaction
Introduction
Diabetes Mellitus is a syndrome with disordered metabolism and inappropriate hyperglycemia due to either deficiency of insulin secretion or to a combination of insulin resistance and inadequate insulin secretion to compensate [1]. Although hyperglycemia is the main characteristic of all form of diabetes mellitus, the pathogenic mechanism by which hyperglycemia arises differs widely. Some forms of Diabetes mellitus are characterized by an absolute insulin deficiency or a genetic defect leading to defective insulin secretion; where as other forms share insulin resistance as their underlying etiology [1]. Recently, there in increasing evidence that an ongoing cytokine induced acute phase response which is sometimes called low grade inflammation, but part of a widespread activation of the innate immune system, is closely involved in the pathogenesis of type 2 diabetes mellitus and associated complications such as dyslipidemia and atherosclerosis. Elevated circulatory inflammatory markers such as C-reactive protein and interleukin-6 predict the development of type 2 Diabetes mellitus and several drugs with anti-inflammatory properties both lower both acute phase reactants and glycemia and possibly decrease the risk of developing type 2 diabetes mellitus. Age, inactivity, certain dietary components, smoking, psychological stress and low birth weight are among the risk factors for type 2 diabetes mellitus, which are also known to be associated with activated innate immunity. Activated immunity may be the common antecedent of developing type 2 diabetes mellitus [2].
Diabetes Mellitus is a syndrome with disordered metabolism and inappropriate hyperglycemia due to either deficiency of insulin secretion or to a combination of insulin resistance and inadequate insulin secretion to compensate [1]. Although hyperglycemia is the main characteristic of all form of diabetes mellitus, the pathogenic mechanism by which hyperglycemia arises differs widely. Some forms of Diabetes mellitus are characterized by an absolute insulin deficiency or a genetic defect leading to defective insulin secretion; where as other forms share insulin resistance as their underlying etiology [1]. Recently, there in increasing evidence that an ongoing cytokine induced acute phase response which is sometimes called low grade inflammation, but part of a widespread activation of the innate immune system, is closely involved in the pathogenesis of type 2 diabetes mellitus and associated complications such as dyslipidemia and atherosclerosis. Elevated circulatory inflammatory markers such as C-reactive protein and interleukin-6 predict the development of type 2 Diabetes mellitus and several drugs with anti-inflammatory properties both lower both acute phase reactants and glycemia and possibly decrease the risk of developing type 2 diabetes mellitus. Age, inactivity, certain dietary components, smoking, psychological stress and low birth weight are among the risk factors for type 2 diabetes mellitus, which are also known to be associated with activated innate immunity. Activated immunity may be the common antecedent of developing type 2 diabetes mellitus [2].
Material and Methods
Study design
Following four groups of subjects were selected for present study:
A. 12 newly diagnosed untreated type 1 diabetes mellitus patients
B. 25 newly diagnosed untreated type 2 diabetes mellitus patients within the age limit of 30-60 years
C. 25 type 2 diabetes mellitus patients who are under treatment of oral hypoglycemic drugs for at least 5 yrs between the age limit of 30-60 yrs.
D. 30 nondiabetic healthy controls.
Height and weight of all subjects were recorded, and body mass index was calculated. None of the ninety-two volunteers were alcoholics or smokers. The participants did not suffer from chronic inflammatory diseases like asthma, chronic bronchitis, and rheumatoid arthritis as was ascertained by clinical history. Informed consent was taken from the individual subjects prior to blood collection. The study was approved by institutional ethical committee.
The following methods were followed for determination of each of the parameters.
Blood glucose estimation
Blood glucose was estimated with Agappe manual diagnostic kit by GOD-POD methodology. The enzymatic procedure of Trinder P was followed [6]. Glucose is oxidized by glucose oxidase to gluconic acid and H2O2. Hydrogen peroxide in presence of phenol and 4-amino antipyrine is acted upon by peroxidase to form red quinine. The absorbance of colored compound was measured at 532nm.
Fibrinogen assay
Fibrinogen assay in plasma was carried out by Biuret method [7]. Fibrinogen present in the plasma is converted to fibrin in presence of calcium chloride. The fibrin clot formed is collected and then digested with NaOH. Protein content of the clot is determined using a red filter. 0.5ml of plasma was mixed with 14 ml of distilled water and 0.5ml of 2.5% calcium chloride solution in a small beaker and incubated at 37ºC until a clot was formed. Then a glass rod was rotated to collect the clot on to it. The rod was pressed against the side of the beaker to squeeze out any solution and to compress the clot. Care was taken to pick up any small piece of clot on the rod, which may have become detached and was dried by pressing carefully against a filter paper. Then it was transferred into a test tube into which the digestion was to be carried out.
After that, the clot was digested with 0.5ml of 0.1N NaOH in a boiling water bath. After cooling, 3.5ml of working Biuret reagent was added to the tube. The OD of the blue color developed was read at 555nm after standing the tube in a water bath at 37 °C for 5minutes. 0.5ml of standard protein solution (800mg/dl) and 0.5 ml of distilled water as blank were treated similarly.
Estimation of serum ceruloplasmin
Ceruloplasmin assay in serum was carried out by the method of Sunderman et al. [8]. At pH 5.4, ceruloplasmin catalyzes the oxidation of PPD to yield a coloured product which is believed to correspond either to Bandrowski’s base or to Weurster’s red. The rate of formation of colored oxidized product is proportional to the concentration of ceruloplasmin, if, a correction is made for the nonenzymatic oxidation of PPD. Therefore, simultaneous assay were carried out with and without sodium azide, which inhibited the enzymatic oxidation of PPD. The difference between the results of the two assays is proportional to ceruloplasmin concentration.
Estimation of α1-antitripsin
α-1 antitrypsin assay in serum was carried out by the modified method described by Sundaresh et al. [9]. The Proteolytic enzyme trypsin hydrolyses casein, with the formation of smaller peptides. The enzyme reaction after suitable interval of time is arrested by the addition of TCA which precipitated the protein, but the peptides are soluble in the acid. The TCA soluble fragments are a measure of Proteolytic activity of the enzyme. When the inhibitor is added to the preincubated mixture, it prevents the release of peptides by the Proteolytic enzymes. Thus, the estimation of TCA soluble components in the presence and absence of inhibitor is a measure of inhibitory activity against Proteolytic enzymes. The TCA soluble fragments are analyzed by the method of Lowry. The final color formed is a result of Biuret reaction of the peptides with copper ions in alkali and reduction of the phosphomolybdic acid reagent by the presence of tyrosine and tryptophan which are present in the treated peptides.
Determination of serum α-1 acid glycoprotein
α-1 acid glycoprotein assay in serum was carried out by the method of Winzler RJ et al. [10]. After removing heat coagulable proteins with perchloric acid, the orosomucoid which remains in the solution is precipitated by phosphotungstic acid and estimated by determining it carbohydrate content by reaction with an orcinol-sulphuric acid reagent, or its nitrogen by Kjeldahl nesslerization or its tyrosine content using FolinCiocalteau reagent.
Result
The anthropometric data of the subjects participated in the study are presented in Table 1.
Discussion
The aim of this study was to examine inflammation as a pathogenetic causeof type 2 diabetes mellitus. Twenty-five type 2 newly diagnosed patients showed increased levels of α1- antitrypsin, α1-acid glycoprotein, ceruloplasmin and fibrinogen. The findings were in agreement with most of the authors who worked with acute phase proteins in type 2 diabetes [11-13]. The role of chronic low-grade inflammation in the pathogenesis of type 2 diabetes seems possible beyond doubt. The course of the disease and resulting complications are similar in both type 1 and type 2 diabetes. The most dreaded complication being that of development of atherosclerosis resulting in cardiovascular diseases. Fibrinogen is identified as an independent risk factor in the development of ischemic heart diseases [14]. Irrespective of the patients being type 1 or type 2, the risk of developing atherosclerosis remains the same. Hence there must be some mechanism which links the pathogenecity of type 1 and type 2 diabetes. Barrazzani et al. [15] infused insulin to non-diabetics, type 1 and type 2 diabetics and studied its role in fibrinogen production. Insulin replacement activity suppressed fibrinogen production in non-diabetics and type 1 diabetic individuals.Fibrinogen production and its plasma concentration increased in insulin resistant type 2 diabetics when euglycemia and euaminoaciduria were maintained. They postulated that an altered response to insulin causes hyperfibrinogenemia in type 2 diabetic patients. If this hypothesis holds well, it doesn’t explain hyperfibrinogenemia in type 1 diabetics where the basic pathology is insulin deficiency. Hence there must be some other factors which stimulate increased fibrinogen synthesis in type 1 patients contributing to cardiovascular disease risk. An insulin resistance syndrome score [16] was developed based on clinical risk factor in patient with type 1 diabetes and validated using euglycemic-hyperinsulinemic clamp studies. Fibrinogen levels were significantly associated with this insulin resistance syndrome score. This may explain high fibrinogen level in type 1 diabetes. But it still does not answer the above findings since the type subjects in this study were newly diagnosed. Hence the mechanism of increased fibrinogen synthesis needs to be proved further.
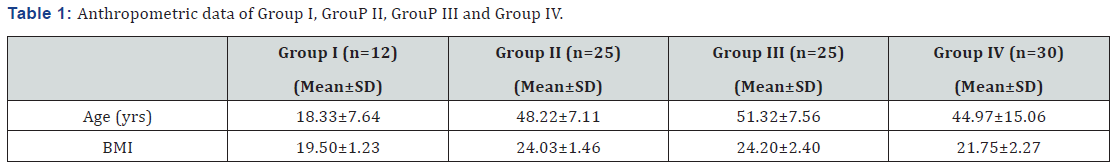
Group I: Type 1 diabetes mellitus patient (newly diagnosed)
Group II: Type 2 diabetes mellitus patient (newly diagnosed)
Group III: Type 2 diabetes mellitus patient (under treatment for at least 5 years)
Group IV: Control
n =Number of Subjects; SD: Standard Deviation; BMI: Body Mass Index
Ceruloplasmin is known to have antioxidant action [17]. It is also an acute phase protein with a response of intermediate magnitude. Ceruloplasmin is known to stimulate cell proliferation and angiogenesis [18]. The higher levels of ceruloplasmin in both in type 1 and type 2 as compared to controls may be due to an oxidative stress that is prevalent in both types of diabetes [19,20]. Ehrenwald [21] showed a very interesting feature of ceruloplasmin. The intact human ceruloplasmin which is 132 KD molecules caused increased oxidation of LDL in vitro. Starkebaum & Harlan et al. [22] also showed that increased serum ceruloplasmin could generate excess oxidized LDL, and cause vascular injury by generating free radicals such as hydrogen peroxide [22]. These findings defined the earlier notions of the antioxidant activity of ceruloplasmin. By further investigations Ehrenwald et al. [21] found that the holoceruloplasmin, which is seen in serum as a 132KD molecule, has a prooxidant effect and the action was attributed to the copper ions present in ceruloplasmin. The commercially available ceruloplasmin is a degraded product containing either 115KD fragment or 19KD fragment or both. These had an antioxidant effect. The works done to show that ceruloplasmin as an antioxidant used these degraded products. The antioxidant action of a commercial ceruloplasmin was observed even in the system where holoceruloplasmin oxidized LDL [23]. Hence considering ceruloplasmin as an antioxidant in vivo is debatable. The LDL oxidizing action of ceruloplasmin could probably explain at least in part of the increased risk of IHD in both type 1 and type 2 diabetes. Also, it could not be wrong to count ceruloplasmin as an acute phase reactant whose levels are increased in both the types of diabetes.
The values of various parameters when compared between the untreated type 1 patients and type 2 patients reveals a significant increase in type 2 patients. Even the ceruloplasmin values, although not statistically significant, were slightly higher in the type 2 patients. The mean random blood sugar (RBS) values in group 1 (Type 1 diabetes) patients was 338.25±50.97mg/ dl and that of group II (Type 2 newly diagnosed diabetics) was 193.26±35.30mg/dl. Despite this huge difference, the inflammatory markers levels were higher in the type 2 patients which go to prove that the glycemic status doesn’t influence the inflammatory markers. This is in accordance with previous findings [24,25]. Evidence is available to say that inflammatory markers are elevated well before the clinical manifestation of hyperglycemia [26-29]. This also gives credence to the thought that activation of innate immunity is not related to hyperglycemia. But research has shown that decreasing plasma glucose levels decrease the concentration of acute phase reactants [30]. Also 2 hrs post load glucose values showed positive correlation with the inflammatory markers in few studies. α1-acid glycoprotein levels remained within normal limits in the type 1 patients whereas significant high levels were seen in the type 2 patients ( p<0.0001). Although both type 1 and type 2 patients showed significant high α1-antitrypsin levels, the comparison between two groups also reached statistical significance (p =0.003) with higher values in type 2 patients. The above findings can be best explained as insulin mediated increased synthesis of the hepatic proteins.
The underlying mechanism for the augmented acute phase response is not well understood and the stimulus for the response is unknown. Many hypotheses have been put forward and these include insulin resistance, obesity, atherosclerosis, other diabetic complications and maladaptation of the normal innate immune response to environmental threats [31-33]. The most widely studied is the association of obesity, insulin resistance, type 2 diabetes and acute phase reactants. It has been shown that adipocytes secret many proinflammatory cytokines in the postprandial state [34-36]. The term ‘diabecity’ has received attention [37] of late for obese diabetics. The ‘common soil’ theory proposed, evaluates the involvement of inflammation in the pathogenesis of diabetes and atherosclerosis. Hyperglycemia and insulin resistance could promote inflammation and inflammation may be a factor linking diabetes mellitus to the development of atherosclerosis. Elevated glucose levels promote inflammation by increasing oxidative stress [38], by the formation of AGEs and increased TNF (kappa B) [39]. In this study, the mean BMI was found to be 19.5±1.23 in type 1 patient and 24.03±1.46 in type 2 patients. No correlation was found between BMI and acute phase reactants. Hence it can be summarized that there could be multiple pathways involved in the activation of the innate immunity system and much work needed to be done to establish either a casual role in the development of mainly type 2 diabetes and could be type 1 diabetes also.
Having demonstrated that there is an inflammatory process going on in type 2 diabetes, we next thought of estimating inflammatory markers in patients on treatment (for at least 5 years) with oral hypoglycemic drugs. Many of the drugs have been shown to have anti-inflammatory effects. Statin drugs inhibits HMG-CoA reductase and prevent atherosclerosis and inhibit the acute phase response by diminishing the deposition of LDL particles rich in cholesterol and phospholipids in macrophages and smooth muscle cells [40]. Statins were found to reduce CRP levels and did not correlate with the reduction of the lipid levels suggesting that the in addition to their ability to reduce LDL, statins may also inhibit the acute phase response [41]. Freeman et al. [40] showed that statin therapy also prevents diabetes mellitus. Pravastatin therapy in the West of Scotland Coronary Prevention Study resulted in a 30% reduction of risk of developing type 2 diabetes. Salicylates in high doses have been known to lower glycosuria in diabetic patients. Although earlier studies were contradictory, these studies have used lower aspirin doses (<3gm/day) and therapeutic duration was only for a few days. Hundal [42] reported that high doses of aspirin (7gm/day) for 2 wks caused 25% reduction in fasting plasma glucose, 50% reduction in triglyceride and 15% reduction of CRP concentration independently of the changes in the plasma insulin concentration. The recently introduced oral hypoglycemic agents thiazolidinedions (Glitazone) are peroxizome proliferators activated receptor γ (PRAR γ) agonist that have been regarded as insulin sensitizes through mechanisms such as altered transcription of insulin sensitive genes controlling lipogenesis, adipocytes differentiation, fatty acid uptake and GLUT 4 ( Glucose Transporter 4) expression. They also have an anti-inflammatory action inhibiting cytokine production, macrophage activation and reducing CRP as well as WBC count in type 2 diabetic subjects [43-46].
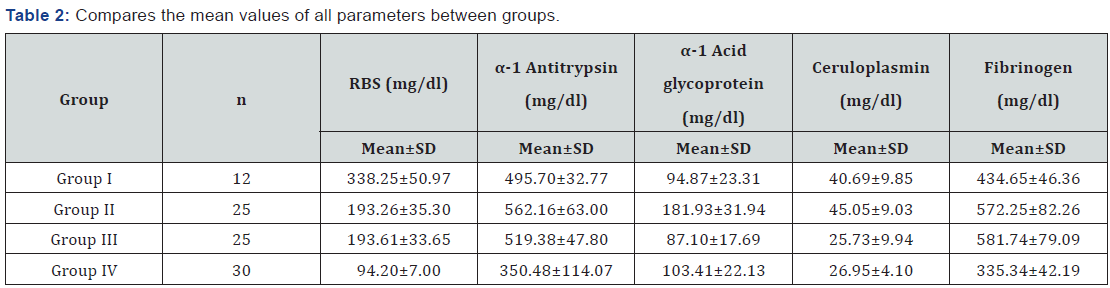
n =Number of Subjects; SD: Standard Deviation; RBS: Random Blood Sugar
Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) are also known to decrease insulin resistance in either type 1 or type 2 diabetic patients with concomitant hypertension [47]. Torlone et al. [48] demonstrated improved glycemic control in patients with arterial hypertension and type 2 diabetes using ACE inhibitors [48]. Insulin has a potent anti-inflammatory activity [32]. Insulin was found to be a rapid nonspecific and dose dependent inhibitors of the cytokine and glucocorticoids stimulation of acute phase protein, gene expression and exerted effect at the transcriptional levels. Insulin inhibition applied to all cell cytokines tested but to various degrees depending upon the particular acute phase gene [49].
In this study, of the 25 type 2 diabetic patients on treatment for at least 5 years, 8 patients were in sulfonylurea-metformin combination, 7 were on glitazone, 6 were on sulfonylurea alone, 2 were on glitazone-metformin combination and 2 were on metformin alone. When compared with newly diagnosed untreated group (Group II) the levels of α1-antitrypsin, α1- acid glycoprotein and ceruloplasmin were statistically lower. No significant difference was found in the fibrinogen levels. The values of α1-acid glycoprotein and ceruloplasmin were comparable to those of the control group. The RBS values were like those of untreated group (193.62±33.65 and 193.26±35.30). It is interesting to note that α1-acid glycoprotein levels were almost the same in type 1 diabetes type 2 untreated patients and control (Table 2). Probably α1-acid glycoprotein is the most amenable acute phase protein to treatment modalities. Comparable ceruloplasmin levels in type 2 patients on treatment and controls again raise the question as to the ‘prooxidant’ or ‘antioxidant’ action of ceruloplasmin. No change in fibrinogen values suggest multiple pathway involvement that are poorly understood.
To Know More About Current Research in Diabetes & Obesity Journal Please click on:
https://juniperpublishers.com/crdoj/index.php
To Know More About Open Access Journals Please click on: https://juniperpublishers.com/index.php


Comments
Post a Comment