Observational Study on the Effectiveness of Treatment with the GLP1-Receptor Agonist Dulaglutide in Patients with Type 2 Diabetes Mellitus: Real-Life Study in Spain
CURRENT RESEARCH IN DIABETES & OBESITY JOURNAL JUNIPER PUBLISHERS
Authored by Amelia Oleaga
Abstract
Dulaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1RA) used in the treatment of Type 2 Diabetes Mellitus which has been shown to be effective in reducing HbA1c and weight with a high degree of safety.
Aims: To evaluate the effectiveness of 1.5mg weekly dulaglutide under condition of routine clinical practice. The safety and discontinuation rate and its cause were quantified. The results obtained were compared with those published in randomised clinical trials and other observational studies.
Subjects and Methods: 208 patients were included in this study, mean age was 62 years, BMI 35.7kg/m2 and HbA1c was 8.48%.
Results: The results showed a statistically significant reduction in HbA1c of -0.95% and in mean weight of -4.63Kg after 24 months follow-up. The drug’s effect was lower in patients who had received previous treatment with another GLP-1RA, and in patients on insulin treatment. Hypoglycaemia was more frequent in the first 6 months of treatment (7.2%), and the treatment discontinuation rate was 31.2%, with lack of efficacy being the most frequent cause.
Conclusions: The results of this study demonstrate the effectiveness and safety of treatment with weekly dulaglutide and are consistent with other multicentre studies conducted under routine clinical practice conditions.
Keywords: Glucagon-like peptide; Glycaemic control; Hypoglycaemia; Hypothalamus; Weight control; Fasting blood glucose; Cholesterol; HDL; LDL; Triglycerides; Creatinine; GFR
Introduction
Despite recent advances that have expanded the therapeutic options available, achieving and maintaining glycaemic control in patients with T2DM continues to be a great challenge. Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are a pharmacological group increasingly in use for the treatment of DM2 [1]. Glucagon-like peptide 1 is an incretin hormone secreted by the L cells of the ileum, which stimulates insulin secretion and suppresses glucagon secretion in ingestion through its effect on the pancreas. In addition, it delays gastric emptying and causes satiety due to its indirect action on the hypothalamus [2]. In this way, GLP-1RA act by causing a reduction in weight and reducing HbA1c levels with a lower hypoglycaemia risk than with sulfonylurea and insulin [3].
Dulaglutide is a long-acting glucagon-like peptide-1 receptor agonist used in the treatment of Type 2 Diabetes Mellitus. The safety and efficacy of dulaglutide has been established in randomized control trials (AWARD studies) [4] but limited real life studies have documented its effectiveness in usual care settings. Additionally, data in Spanish population are scarce. Moreover, there are limited data on switching from another GLP1-RA and outcomes comparing patients on insulin with insulin naive patients. The aim of this study was to evaluate the effect of 1.5mg weekly dulaglutide on glycaemic and weight control and the safety of the drug in adults with T2DM in a real-life experience, explore the differences when switching from another GLP-1RA, and compare our results with those published in randomised clinical trials and other observational studies.
Material and Methods
Study population
A retrospective observational study was carried out under conditions of routine clinical practice in patients with T2DM at Basurto University Hospital, Bilbao, Spain. Medical records of 300 patients whom dulaglutide was prescribed since its launch (December 2015) were reviewed, and a total of 208 patients were included. Data were obtained by searching in the pharmacy database of the Bilbao-Basurto health service organisation. The study protocol was approved by the organization’s Research Ethics Committee.
Description of variables
Patient characteristics were recorded at baseline (first visit when Dulaglutide was prescribed) and every six months until 24 months of follow-up. Data recorded in the initial visit included gender, age, year of diagnosis of DM2, presence or absence of retinopathy, macrovascular complications, background antidiabetic medication, previous use of GLP1-RA other than Dulaglutide, antihypertensive treatment, statins and previous CV event. To measure the efficacy of the drug during the follow-up period, the evolution of the following parameters was analysed: height, weight, BMI, fasting blood glucose (FBG), HbA1c, albumincreatinine ratio, cholesterol, HDL, LDL, triglycerides, creatinine and GFR. To assess the safety of the drug, the frequency of hypoglycaemia was measured, which was categorised into weekly, monthly and occasional hypoglycaemia. Causes that led to treatment withdrawal were recorded and classified as gastrointestinal disorders (nausea, vomiting, diarrhoea, abdominal pain), lack of efficacy (not observing improvement in HbA1c, or weight loss), skin reactions, the patient’s own choice, BMI <30 and other causes.
Statistical analysis
Statistical analysis was performed using the SPSS 20.0 program. For the analysis of qualitative variables, frequencies were expressed in percentages (%), and for the quantitative variables, the mean and standard deviation were analysed. To prove that continuous variables followed a normal distribution, the Kolmogorov-Smirnov test was carried out. The analytical results include follow-up checkpoints, carried out at 6, 12, 18, and 24 months of treatment. Some data were collected at 36 and 48 months, but as there were few data, they were not included in the analysis. A 95% confidence interval was set, and the allowed alpha error was 0.05 for statistical significance (p <0.05). The comparison of quantitative variable means was carried out using Student’s T test, and the study of paired variables was necessary in the case of HbA1c, weight, fasting blood glucose and BMI. For qualitative variables, the χ2 test was used.
Results
The clinical data corresponding to 208 patients who had received treatment with 1.5mg weekly dulaglutide were reviewed. The mean follow-up time was 20 months (6-49 months). Table 1 shows the baseline characteristics of the studied population. Of the 208 patients studied, 109 were men (52.4%) and 99 women (47.6%). The mean age of the entire sample was 62.57±9.86 years, the mean duration of DM2 was 13.14±7.35 years, HbA1c: 8.48±1.63%, Weight: 98.08±17.49Kg, BMI: 35.75±5.26Kg/m2 and FBG: 180.35±63.61mg/dl. 64 (30.8%) of the patients had previously used a GLP1-RA and 38% were on insulin treatment.
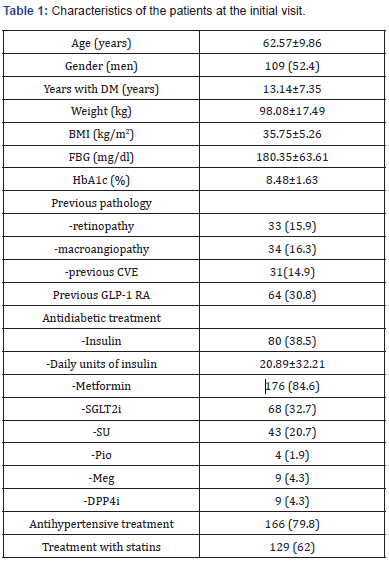
Data presented as sample mean ± standard deviation or as n (%). DM: Diabetes Mellitus; BMI: Body Mass Index; FBG: Fasting Blood Glucose; HbA1c: Glycosylated Haemoglobin; CVE: Cardiovascular Event; GLP- 1 RA: Glucagon-Like Peptide-1 Receptor Agonist; SGLT2i: Sodium- Glucose Transported Type 2 Inhibitors; SU: Sulphonylureas; Pio: Pioglitazone; Meg: Meglitinide; DPP4i: Dipeptidylpeptidase 4 Inhibitors.
Evolution of weight, BMI, Hba1c and fasting glucose
Table 2 & Figure 1 show the evolution of weight, BMI, HbA1c and FBG of the patients studied at each follow-up visit. In patients who completed 24 months of treatment, a progressive weight reduction was observed from baseline to 24months (4.63±8.09; p<0.001). A decrease was also seen in BMI (1.73±2.99 kg/m2; p<0.001). HbA1c showed a statistically significant decrease of 0.95% from baseline to 24months (8.55% vs 7.59%; p <0.001). However, the greatest reduction was seen at 6 months of treatment (1.32±1.53%) reaching a mean level of 7.16±1.31% at the end of the study.
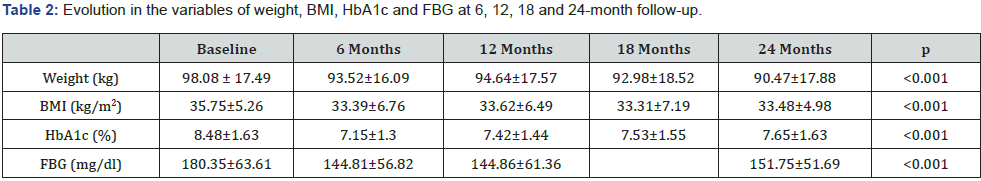
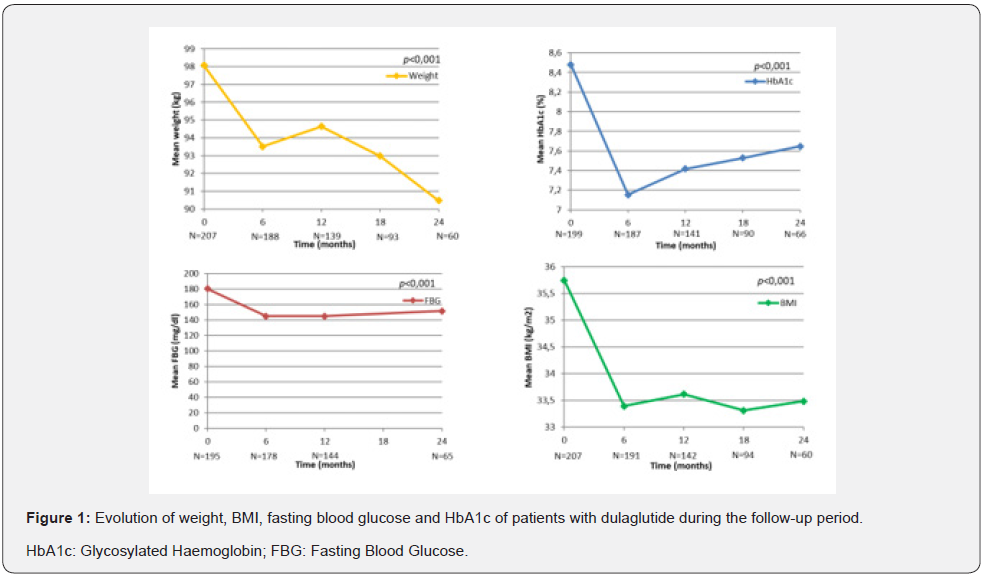
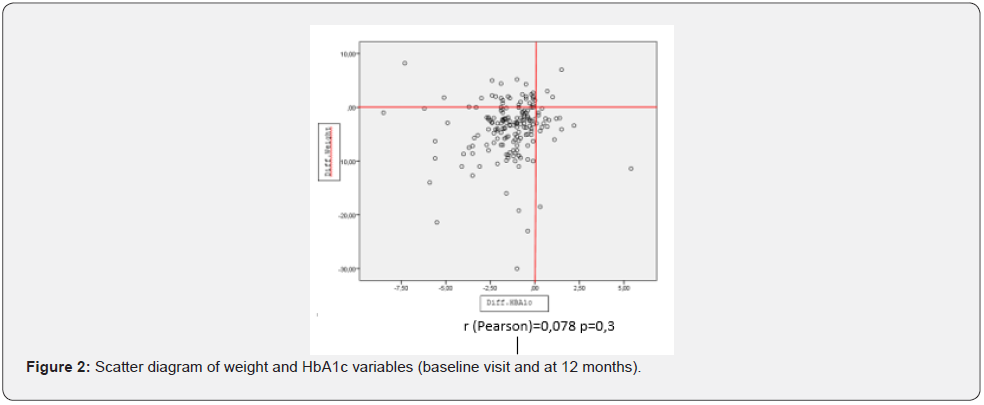
At the baseline visit, 20% of the patients had an HbA1c ≤7%; 54% after 6months, 43% after 12 months and 36% after 24 months. Fasting blood glucose levels were significantly reduced after 6 months to 145.01±56.63mg/dl, although they rose slightly after 24 months to 152.47±51.78mg/dl. With regard to BMI, 38.1% of the patients had grade 2 obesity (BMI ≥35kg/m2). 28% of the patients achieved a BMI of less than 30 by month 24 of follow-up. The data on the weight loss and HbA1c decrease of 139 patients who completed one year of treatment were analysed, and it was observed that most of the patients achieved a concomitant decrease in weight and HbA1c, as can be seen in the scatter diagram (Figure 2).
Results based on previous use of GLP-1 RA
In patients who had not received prior treatment with a GLP-1 RA, a mean weight loss of 9.18kg (99.23±18.15 vs 90.05±19.74) was obtained after 24 months. Weight loss was lower in the group previously treated with GLP-1 RA (4.28kg) (95.42±15.68 vs 91.14±14.79). A statistically significant greater reduction in HbA1c was observed at 6-month follow-up in the group of patients not previously treated with GLP-1 RA (-1.57 vs -0.76%; p <0.01). However, HbA1c reached similar levels over time in both groups.
Results based on insulin treatment
Insulin naive patients had a higher weight at each follow-up visit. Weight loss was similar in both groups. When comparing mean HbA1c based on insulin treatment at the different study follow-up points, higher levels were seen in patients on insulin treatment. Insulin naive patients achieved greater reduction in HbA1c during follow-up. (-0.9 vs -0.5, p=0.03). Background antidiabetic medication was adjusted according to clinical criteria during the study. A statistically significant improvement was observed in all the parameters of the lipid profile, except in HDL levels from the beginning to the end of the study. There were no differences in renal function parameters (Table 3). Hypoglycaemia was classified into occasional, monthly and weekly hypoglycaemia. The highest percentage of hypoglycaemia was recorded at 6 months (7.2%), was occasional, and mainly in patients on basal insulin, sulfonylureas or glinides, falling to 1.4% at 24 months.
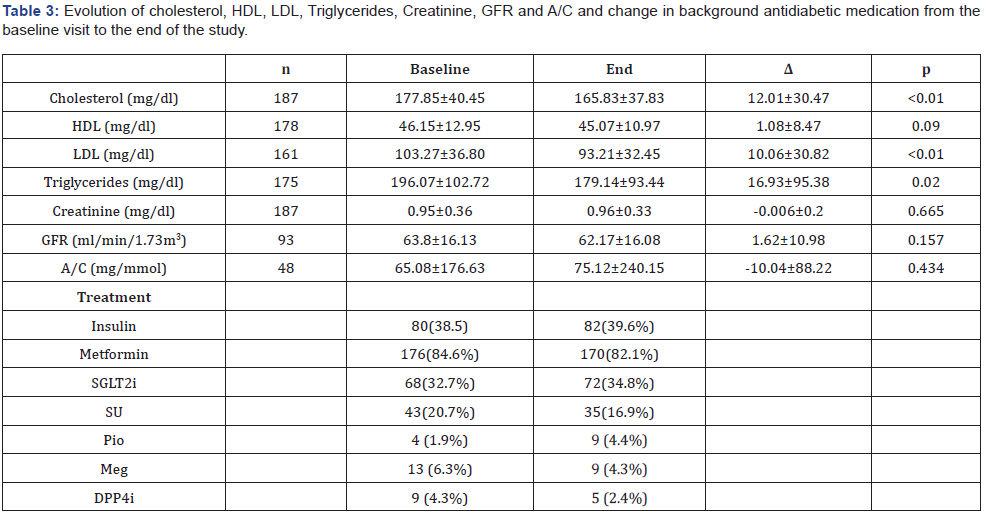
Data presented as sample mean ± standard deviation. HDL: High-Density Lipoproteins; LDL: Low-Density Lipoproteins; GFR: Glomerular Filtration Rate; A/C: Albumin Creatinine Ratio.
Discontinuation of Treatment
65 of the patients studied (31.2%) discontinued treatment with dulaglutide for different reasons, including gastrointestinal alterations (11.44%), lack of efficacy (13.46%), skin reactions, the patient’s own choice, BMI <30, and other causes. When comparing the frequency of discontinuation of therapy based on the previous use of another GLP-1RA, 40% of the patients who had used a previous GLP-1 RA discontinued dulaglutide, while 25.9% of the patients who had not used any GLP-1RA before, did. (p=0.049). Lack of efficacy was the most frequent cause of withdrawal in both groups.
Discussion
The main objective of this study was to assess the effectiveness of weekly dulaglutide in patients with DM2, under normal clinical practice conditions, evaluating its effect on weight, BMI, HbA1c and fasting blood glucose. The demographic and clinical characteristics of the patients included in this study, resemble those of the population included in the real word study in Spain with Dulaglutide published by Moreno et al. [5], in which initial weight, HbA1c and fasting glucose were similar to those of our patients. Also comparable to other real-life studies with various GLP-1 RAs [1,5-8], and to the profile of the patients included in the multicentre cross-sectional study carried out in Spain (CHADIG) [9]. Both BMI (35.75kg/m2) and initial HbA1c (8.48%) showed very similar levels to those of the CHADIG study. Nevertheless, the history of DM among the patients in the present study was longer (13 vs 9 years). Moreno et al. [5], don’t refer the duration of DM2. However, baseline characteristics of our patients were different from those subjects included in the dulaglutide development clinical programme (AWARD). Our patients were older, more obese, and with a longer duration of DM, although baseline HbA1c was quite similar (8.08 vs 8.48) [4].
The findings of this observational study in patients with DM2 confirm the effectiveness and safety of treatment with weekly 1.5mg dulaglutide under normal clinical practice conditions. The data showed a reduction in HbA1c of -0.95% and a decrease in mean weight of -4.63 Kg after 24 months. In addition, 20% of our patients had an HbA1c <7% at the beginning of the study, so they would not have been candidates for clinical trials, where generally, HbA1c must be above 7.5%. This may explain the lower reduction in HbA1c observed in our study compared to that obtained in clinical trials (1-1.6%). The decrease in HbA1c from baseline levels was -1.32% at 6 months and 1.17% after 12 months, similar to that observed in the AWARD trials, in which a decrease greater than -1% was shown, reaching levels of -1.5% and -1.6% in AWARD-1 and AWARD-4 respectively.
The effect on the decrease in HbA1c was at a maximum at 6 months, and although the significant reduction in HbA1c was maintained from the baseline, it seems that the effect was slightly lost. This contrasts with data obtained by Moreno et al. [5], in which the sustained reduction in HbA1c was observed after 24 months of follow-up, as well as in the study by Tofé et al. [1] in which the decrease in HbA1c was seen to be maintained after 36 months. However, Unni et al. [8] in a 6-month study with 2,465 patients undergoing treatment with weekly exenatide, dulaglutide or albiglutide reported a decrease in HbA1c of 0.5%, clearly lower than that obtained both in our study and in other real-life studies and clinical trials, attributing the differences to likely lower adherence in real life. However, most of the patients included in the Unni study were under weekly treatment with exenatide, a molecule that has shown less efficacy than others have. In contrast, our study showed an increase in the number of patients (36%) who achieve an optimal degree of DM control (<7%) by the end of the follow-up period.
With regard to BMI, the patients in our study had a mean initial BMI of 35.75kg/m2, which was clearly higher than the average BMI of the patients included in clinical trials of 32-33kg/m2 (T [1]. These aspects are probably related to the different characteristics of the population whom the drug is prescribed in clinical practice, where more importance is placed on the patient’s weight rather than on the hypoglycaemic effect of the drug. The observed weight reduction was -3.89kg after 6 months and -4.63kg after 24months, this reduction being greater than that seen in the phase 3 clinical trials of the AWARD program, in which average weight loss was 1.5-3kg, although starting from a lower baseline weight [10]. In relation to other real-life studies, Moreno et al. [5], observed a weight reduction of 7kg after 24 months, which was higher than that obtained in our study. A possible explanation for these differences could be the already known fact that the greater the initial weight of the population, the greater the weight reduction achieved. Regarding the change in weight, 28% of patients went from obese to overweight, which could likely confer substantial benefits on their health. Fasting blood glucose showed a similar trend, with a significant fall of -34.87mg/dl after 6 months, which was comparable to those obtained both in the real-life study by Moreno et al. [5], and in the AWARD clinical trials.
This study, in contrast with other published studies, evaluated changes in weight and HbA1c based on whether the patients had received previous treatment with another GLP-1RA. Both weight and HbA1c reduction were found to be less in patients who had received prior treatment with another GLP-1 RA. This can be attributed to the fact that both parameters showed lower baseline values in this group of patients, although the lower effect observed in patients previously treated with other GLP-1 RAs might also reflect a lower therapeutic response due to the effect on the same pathophysiological mechanism. Additionally, the evolution of weight and HbA1c were compared depending on whether or not the patients received concomitant insulin treatment. Patients on insulin treatment lost less weight than insulin naive patients (7.42kg vs 8.35kg), but this could be explained by the fact that the latest had higher baseline weight levels, although the negative effect of insulin on weight loss is well known and could be partially responsible for this difference. Additionally, the lesser reduction in HbA1c in patients on insulin treatment (-0.52% vs -0.9%) probably reflects a stage of more advanced diabetes and the need for a greater complexity of treatment in these patients.
The most frequent use of GLP-1 RAs reported in Spain is in combination with an oral antidiabetic agent, followed by association with basal insulin [11], as shown in the present study. In our patients, dulaglutide was combined most frequently with metformin (84.6%), followed by SGLT-2i (32.7%) and sulfonylureas (20.7%). It is noteworthy the high percentage of patients on SGLT- 2I, 32.7% of the population, higher than those on sulfonylureas, reflecting the trend towards a different approach to the disease in the last years. The frequency of hypoglycaemia after 6 months of treatment in the present study was 7.2%, falling to 1.4% at the end of the study. The slight increase in hypoglycaemia observed at 6 months, compared to the baseline, could be related to the lack of adjustment of concomitant treatments (insulin, secretagogues) in this first period. This low prevalence of hypoglycaemia confirms the safety of the drug, as has been shown in other studies [1,5].
In our study, the treatment drop-out rate was 31.2%, higher than that observed in the studies by Moreno et al. [5],(9%) and Mody et al. [12], which described a higher persistence rate with dulaglutide compared to liraglutide and a discontinuation rate of 20%, but rather similar to the study by Mody & Tofé et al. [1,13], which reported 37%, and in which they attribute most discontinuation to lack of efficacy, although it must be noted that in the work of Tofé et al.[1], most of the patients were on weekly exenatide and only a small percentage on dulagutide. In our study, the most frequent cause of discontinuation of treatment was also lack of efficacy (13.46%), accounting for almost half of the dropout cases, but this proportion was not particularly high and did not differ much from the proportion of patients in whom treatment was withdrawn due to gastrointestinal adverse effects (11.54%). The discontinuation rate observed is higher than that reported in AWARD programme, and probably related to a closer follow-up of patients in clinical trials.
The treatment dropout rate in patients who had received a GLP-1 RA previously was higher than in patients who had not received one (40% vs 25.9%), probably due to the fact that they shared the same pathophysiological mechanism, with the lack of efficacy cited as the most frequent cause of dropout in both groups. Since this work is a retrospective observational study, some limitations must be taken into account when interpreting the results. It is a retrospective design study carried out in a single centre, so the results obtained may not be representative of the general population. Moreover, changes in the patients’ lifestyles were not taken into account, so these could induce some bias in the results.
In conclusion our results demonstrate that weekly 1.5mg dulaglutide, added to standard therapy, reduce HbA1c and body weight without significant hypoglycaemia, in a population of obese patients with long-standing T2DM in conditions of routine clinical practice. The effect of the drug is reduced in patients who had received previous treatment with another GLP-1. Lack of efficacy was the most frequent cause of discontinuation of treatment. Therefore, more real-life studies are needed, especially now that higher doses have received approval for its use in patients with T2D.
Acknowledgments
The study was funded by the Institution on the basis of clinical daily practice, without additional external funding. “Eli Lilly and Co. has supported the medical writing and publication fees of this study without being involved in any steps of its development (conception and design, analysis or interpretation of the results, etc)”.
For More articles in Current Research in Diabetes & Obesity Journal Please click on: https://juniperpublishers.com/crdoj/index.php
For more about Juniper Publishers please click on: https://juniperpublishers.com/video-articles.php


Comments
Post a Comment