Nampt: Intracellular and Extracellular Influence on Diabetes and Obesity
CURRENT RESEARCH IN DIABETES & OBESITY JOURNAL JUNIPER PUBLISHERS
Authored by Kevin G Culligan
Abstract
The prevalence of Type 2 Diabetes Mellitus (T2DM) as a result of increased Obesity, or disability is increasing rapidly. However, only a relatively small percentage of obese individuals develop T2DM, suggesting the contribution of additional factors to whole body insulin resistance. Pharmacological intervention with several anti-diabetic agents, such as Thiazolidinediones (TZDs) can cause increased weight gain, but remain the only option for the majority of obese individuals. A subset of bioactive peptides released from white adipose tissue, termed adipokines, forms the molecular link between obesity and T2DM, conveying whole body insulin resistance and desensitization. Once such adipokine, Nampt, is a rate-limiting enzyme in the production of NAD+. Nampt, also known as Visfatin or PBEF, plays key role in regulation of intracellular redox reactions through the controlled production of NAD+. This production of NAD+ within adipocytes activates downstream enzymes such as SIRT1. Through activation of SIRT1, whole body insulin resistance is prevented by increased Adiponectin release, as well as release of Nampt extracellularly. SIRT1 also inhibits the activation of PPARY-dependent insulin-resistance genes. As a result, the Nampt-NAD+-SIRT1-PPARY axis therefore may offer a novel target for treatment strategies of T2DM.
Keywords: Nampt; Visfatin; PBEF; NAD+; Obesity; Metabolic disorders; SIRT1; PPARγ; Adiponectin; NF-ᶄB
Introduction
Obesity is a medical condition relating to accumulation and storage of excess body fat, predisposing to negative health implications. The fundamental cause of obesity is a simple energy imbalance between calorific intake and calorific expenditure. Sedentary lifestyle and an increase in consumed calories are the main contributors to this increase in weight gain [1]. Such is the tightness of the molecular link between obesity and Type 2 Diabetes Mellitus (T2DM), some researchers utilize the term Diabesity in reference to both [2]. T2DM, the most common form of diabetes, is characterized by
A. A lack of insulin due to dysfunctional pancreatic beta cells.
B. Reduced insulin sensitivity.
C. Insulin resistance [3].
Insulin-resistant individuals demonstrate an impaired ability of insulin to stimulate glucose uptake into skeletal muscle, to suppress gluconeogenesis, and to suppress hydrolysis of triglycerides into fatty acids in adipose tissue.
Adipose tissue has long been considered as a storage depot for triglycerides. However, it has become well established that a secretary role exists for adipose tissue [4]. As well as releasing lipids, cytokines such as Tumor Necrosis Factor-a and interleukins, chemokines such as Monocot Chemo attractant Protein 1 (MCP-1) and coagulation factors such as Plasminogen Activator 1 (PAI-1) and adipokines are also secreted [5]. A subset of chemokines specific to adipose tissue, termed adipokines is also released [6]. Some, such as lepton and adiponectin have been highly characterized and their functions clearly elucidated [7,8].
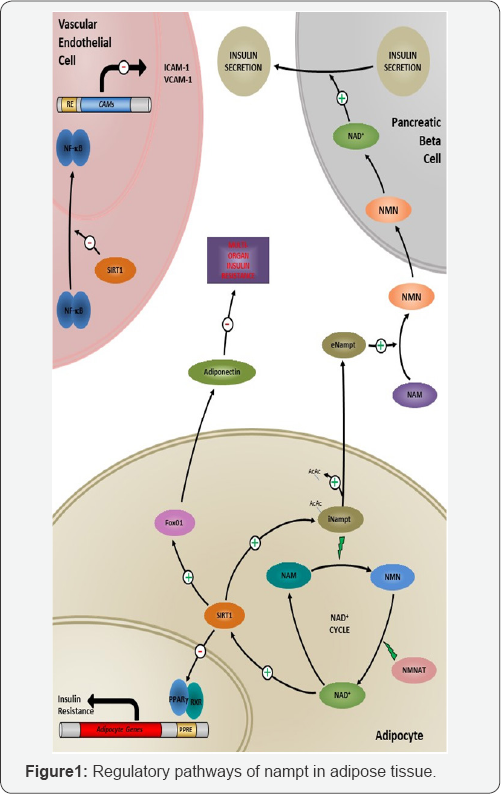
Specifically within adipose tissue, Nicotinamide Adenine Dinucleotide (NAD+) and its derivatives act as essential coenzymes in cellular redox reactions in all living organisms [9]. The primary function of the NAD+ pathway mediates energy metabolism, reductive biosynthesis and anti-oxidation. During cellular redox reactions, the associated coenzymes Nicotinamide (NAM) and Nicotinamide Mononucleotide (NMN) transfer electrons throughout the pathway. In general, NAD+ has been shown to be synthesized by two separate paths; de novo synthesis from tryptophan or through the salvage pathway, being formed by the recycling of the other coenzymes Nicotinamide and NMN [9,10]. NAD+ has also been shown to modulate the activity of essential regulators of cellular longevity [11]. As well as its role in redox reactions, NAD+ is also an important signaling molecule, being released from cells such as adiposities into systemic circulation [12]. Figure 1 Cyclic production process of NAD+ is regulated by the rate-limiting enzyme Nampt. NAD+ can activate SIRT1, which can elevate serum levels of Adiponectin through activation of Fox01. NAD+ inhibits the transcription activity of PPARY, preventing the transcription of adipocytic genes involved in insulin resistance. SIRT1 can also deacetylate iNampt, releasing it extracellularly. Once released, eNampt can convert Nicotinamide (NAM) to Nicotinamide Mononucleotide (NMN), and generate NAD+ in pancreatic beta cells, modulating insulin secretion. The presence of SIRT1 in vascular endothelial cells can regulate expression of cell adhesion molecules, implicated in the vascular pathogenesis of Type 2 Diabetes Mellitus.
Nampt
Nicotinamide phosphoribosyltransferase (Nampt) is a regulatory enzyme of the NAD+ cascade synthesized and released by adiposities, as well as inflammatory cells such as activated macrophages [13]. The enzyme was originally identified as presumptive cytokine termed Pre-B-cell colony-Enhancing Factor (PBEF), due to its isolation and cloning from activated lymphocytes [14]. Visfatin, referring to visceral fat from which the enzyme is derived was separately isolated and shown to possess insulin-mimetic effects, being predominantly secreted from visceral fat [12]. Later, amino acid sequence analysis of Visfatin showed sequence identity to PBEF/Nampt [15].
While all three names (Nampt, PBEF, and Visfatin) can be found used interchangeably in the literature, both the HUGO Gene Nomenclature Committee (HGNC) and the Mouse Genomic Nomenclature Committee (MGNC) have approved the use of Nampt as the official nomenclature of the gene and the gene product. Therefore, Nampt will be used throughout this review.
Genetic Location
Located on the long arm of chromosome 7(7q22.1-7q31.33) the NAMPT gene is well conserved across species [16]. The gene encodes a 2.4kb mRNA sequence, giving rise to a 491amino acid 52 kDa protein [15]. Interestingly, although Nampt is believed to be secreted, the mature protein sequence lacks both a signal sequence and capsize cleavage site [12]. The mature Nampt protein forms a homodyne, which is essential for catalytic activity of the enzyme. Homodimerization results in the formation of two active sites at the interface of the dimeric protein. It is believed that this region is essential for regulation of the production of NMN from nicotinamide [15].
Types and Regulation
The mature protein of Nampt exists in two different cellular locations: intracellular Nampt (iNampt) and extracellular Nampt (eNampt) [17]. Although both forms of Nampt are critical rate- limiting enzymes in the production of NAD+, their rate of activity differ, depending on cellular location. In adipose tissue, iNampt is involved in the salvage pathway in the production of NAD+ [13]. Within adipose tissue, NAD+ is reduced and oxidized continuously. iNampt converts Nicotinamide to NMN, and is the rate limiting enzyme in this step [18]. Subsequently, NMN becomes acetylated by NMNAT, converting it to NAD+. During redox reactions in the cell, NAD+ consumers utilize NAD+, converting it to Nicotinamide, which in the salvage cycle becomes available for iNampt to reconvert Nicotinamide to NMN. Depletion of the cellular pool of NAD+ is prevented by de novo synthesis of NAD+ from tryptophan [10]. The biological role of eNampt has been a matter of much debate. Initially, it was first believed that the systemic release of eNampt was due to dying cells [19]. Later though, it was demonstrated that the secretion of eNampt occurs from adiposities by secretion through a non-classical secretary pathway [20]. The sequence of Nampt lacks both a signal sequence and caspase cleavage site [21]. SIRT1, one such NAD+ consumer, deacetylates iNampt, releasing it into the extracellular milieu [22]. Once secreted, eNampt is believed to play several roles essential to glucose homeostasis [20].
It has been demonstrated that eNampt can initiate a dose- dependent up regulation of pro- and anti-inflammatory cytokines [23]. With eNampt itself believed to play a role as both a cytokine and adipokine. eNampt mediates the biosynthesis of systemic NAD+. This NAD+ has been shown to be necessary for pancreatic beta cell function, as well as the regulation of Glucose-Stimulated Insulin Secretion (GSIS) [20].
Downstream Mediators
SIRT1
SIRT1, a class III his tone deacetylase, belongs to the family of Sit-ins (SIRTs) [24,25]. Through the consumption of NAD+, SIRT1 deactivate downstream proteins on specific lysine residues [26]. Blocking of SIRT1 activity by techniques such as antisense technology can induce adipose tissue infiltration, as well as infiltration of activated macrophages [26]. Conversely, deactivation of Fox01 by SIRT1 in adiposities has been shown to increase levels of Adiponectin, an adipokine conveying sensitivity to insulin. As a result, the pharmacological activation of SIRT1 by agents such as resveratrol is being investigated as a method for increasing insulin sensitivity in diabetic patients [25].
PPARγ
PPARY has long since been a target for investigation for the treatment of obesity-associated insulin resistance, due to its ability to regulate whole-body insulin sensitivity. Interestingly, in adipose tissue SIRT1 suppresses the actions of PPARY, preventing adiposeness [27,28]. The actions of SIRT1 on PPARY occur at two distinct sites at a post-translational level. Firstly, deactivation of PPARY suppresses PPARY-dependent insulin-resistance genes [27]. Secondly, through inhibition of phosphorylation of ser273 in PPARY, a subset of PPARY-dependent genes are silenced; these genes controlling glucose metabolism and insulin sensitivity [29].
NF-ᶄB
A potential role of eNampt in the pathogenesis of vascular inflammation in obesity and T2DM has been suggested [30]. An induction of adhesion molecules such as ICAM-1 and VCAM-1 in response to eNampt has been demonstrated in leukocytes [31], mediated through the induction of the pro-inflammatory transcription factor Nuclear Factor-k,B (NF-k,B). Induction of NF-k,B-mediated matrix metalloproteinase's (MMPs) in vascular endothelial cells was also demonstrated [32]. This suggests a role for eNampt in the development of the vascular complications associated with obesity and T2DM.
Nampt in T2DM
The development of Nampt knockout mice has supplied evidence of the role of Nampt in both obesity and diabetes. Homozygous deletion of the Nampt gene results in lethality in knockout mice [33]. However, in heterozygote’s, impaired glucose tolerance is seen as a result of the regulation of GSIS by Nampt [20]. Loss of Nampt modulates the levels of NAD+ in pancreatic beta cells, reducing insulin secretion in response to plasma glucose [33].
In the elderly, T2DM is associated with a progressive decline in pancreatic beta cell function [34]. SIRT1 activation by Nampt- generated NAD+ has been implicated in the processes of longevity. Given that SIRT1 is known to positively regulate GSIS, the loss of Nampt through progressive decline in pancreatic beta cell function is believed to contribute to obesity-associated multi-organ insulin resistance [20].
Nampt in Obesity
In adipose tissue, synthesis of NAD+ by Nampt has been shown to be highly sensitive to nutritional changes. These alterations in calorific input have subsequently been implicated in the path physiology of modification of whole-body glucose metabolism and insulin sensitivity [35]. For example, in mice, feeding on hyper calorific diets has been shown to decrease NAD+ levels, primarily as a result of a reduction in Nampt [36,37]. Correlation of plasma eNampt levels in human metabolic disorders such as obesity still remains unclear. One of the main reasons for this is a lack of sensitivity of the assay type used to determine serum concentrations [38]. However, a positive correlation between an increase in BMI and Nampt levels has been demonstrated, as well as increased white adipose tissue. Interestingly, gastric banding has been shown to reduce circulating eNampt levels [39]. As assays become more sensitive, more reliable measurement of serum eNampt should give us insight into its effects on metabolic disorders.
Nampt Treatment Strategies
The use of PPARY agonists has been a mainstay treatment in T2DM in non-obese patients for a considerable time. Activation of PPARY by Thiazolidinediones (TZDs) results in the expression of a number of genes involved in both lipid and glucose metabolism and preadipocyte differentiation. TZDs increase insulin sensitivity, as well as increase utilization of glucose by peripheral tissues [40]. TZDs however have been known to cause hepatic damage, as well as adversely inducing an increase body weight, making them unsuitable for use in obese patients [41].
Since Nampt has been shown to positively regulate PPARY through NAD+-mediated activation of SIRT1, interest has grown in the targeting the Nampt-NAD+-SIRT1-PPARY axis as a novel approach in the treatment of age-related diseases such as ageing and T2DM. A naturally-occurring polyphenolic supplement Resveratrol putatively activates SIRT1 [42]. In animal studies, Resveratrol administration was shown to increase insulin sensitivity and increase longevity of mice on high-calorie diets [43]. More selective and potent SIRT1 activators known as STACs (Synthetic Sit-in-Activating Compounds) such as imidazothiazole are currently under investigation [44].
Given the actions of SIRT1 on glucose homeostasis, an interesting therapeutic approach would be in the development of Nampt-NAD+ activating compounds. Since activation of SIRT1 is solely dependent on Nampt-NAD+, small molecule activators of these proteins may prove an effective strategy in the treatment of age-related metabolic disorders.
To More articles in Current Research in Diabetes & Obesity
Journal Please click on:
https://juniperpublishers.com/crdoj/index.php
For more about Juniper Publishers please click on: https://juniperpublishers.com/video-articles.php



Comments
Post a Comment